

The number of electrons in an atom equals the number of protons in the nucleus.The atom is composed of a positively charged nucleus and negatively charged electrons.The atom is the smallest particle of an element that has the chemical properties of that element.Thomson’s atomic model is based on the following postulates: They also produce a lot of heat, which can be dangerous. CRTs are no longer used in televisions and computers because they are not very energy-efficient.The phosphor glows when it is hit by the electrons, creating a picture. They work by firing a beam of electrons at a phosphor-coated screen. Cathode ray tubes were the first type of display to be used in televisions and computers.In this equation, y is the line’s y-coordinate, x is the line’s x-coordinate, a is the line’s slope, and b is the line’s y-intercept. The cathode ray tube experiment is a physics experiment that uses a cathode ray tube to investigate the nature of electric charges and the electric field. The phosphor coating glows when struck by the electron beam, creating the image on the screen. These electrons are then accelerated towards a phosphor-coated screen. The electron beam is produced by heating a cathode, which causes electrons to be emitted. The Cathode Ray Tube (CRT) is a vacuum tube that uses an electron beam to produce a visual display. The cathode rays are used in a variety of applications, including x-rays and television screens. They are a stream of negatively charged particles that are accelerated towards the anode.
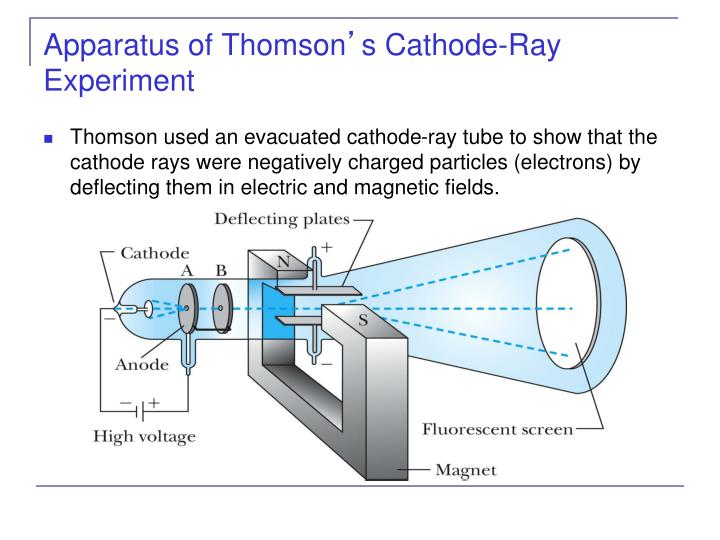
is of one and the same kind this matter being the substance from which all the chemical elements are built up.Cathode rays are a type of radiation that are emitted from the cathode of a vacuum tube. Homson boldly announced the hypothesis that "we have in the cathode rays matter in a new state, a state in which the subdivision of matter is carried very much further than in the ordinary gaseous state: a state in which all matter. "We have in the cathode rays matter in a new state." Measurement of the value of the charge ( e) and confirmed this The proof was far from conclusive.īut experiments by others in the next two years yielded an independent That if cathode rays were particles they had to have a mass very much Experimenting on how cathode rays penetrate gases, he showed (as compared with a charged atom), or else they were amazingly light relativeĬhoice between these possibilities was settled by Philipp Either the cathode rays carried an enormous charge To be far smaller than that of a charged hydrogen atom-more than one Just as Emil Wiechert had reportedĮarlier that year, the mass-to-charge ratio for cathode rays turned out He collected data using a variety of tubes and using different gases. From this data he could calculate the ratio of the mass of a particle to its electric charge ( m/ e). Although he couldn't measure directly the mass or the electric charge of such a particle, heĬould measure how much the rays were bent by a magnetic field, and how much energy they carried.

Homson's third experiment sought to determine the basic properties of the particles.

Homson concluded from these two experiments, "I can see no escape from the conclusion that are charges of negative electricity carried by particles of matter." But, he continued, "What are these particles? are they atoms, or molecules, or matter in a still finer state of subdivision?" To test this idea, he took great pains to extract nearly all of the gas from a tube, and found that now the cathode rays did bend in an electric field after all. Thomson suspected that the traces of gas remaining in the tube were being turned into an electrical conductor by the cathode rays themselves. A charged particle will normally curve as it moves through an electric field, but not if it is surrounded by a conductor (a sheath of copper, for example). Ll attempts had failed when physicists tried to bend cathode rays with an electric field. As Thomson saw it, the negative charge and the cathode rays must somehow be stuck together: you cannot separate the charge from the rays. The electrometer did not register much electric charge if the rays were bent so they would not enter the slit. He found that when the rays entered the slit in the cylinders, the electrometer measured a large amount of negative charge. Thomson wanted to see if, by bending the rays with a magnet, he could separate the charge from the rays. Perrin had found that cathode rays deposited an electric charge. These cylinders were in turnĬonnected to an electrometer, a device for catching and measuring electrical charge. Irst, in a variation of an 1895 experiment by Jean Perrin, Thomson built a cathode ray tube ending in a pair of metal cylinders with a slit in them. He advanced the idea that cathode rays are really streams of very small pieces of atoms.


 0 kommentar(er)
0 kommentar(er)
